





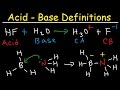














To find conjugate base you have to remove H+ from the given species. Hint: CBR (Conjugate Base Remove H+) So conjugate base of HSO4- is SO4^-2. To find conjugate acid you have to add H+ to the given species. Hint: CAA (Conjugate Acid Add H+) So co... SO_4^(2-) Although it has a negative charge, it will never accept a H^+ to form H_2SO^4(sulfuric acid) . That is because sulfuric acid is a strong acid and completely disassociates in water. Therefore, the sulfate ion (SO_4^(2-)) is the conjugate base of HSO_4^-. In order to get the conjugate acid of any base, add H+ to the base. Accordingly, the conjugate acid of HSO4- is H2SO4. Also, HSO4- is the conjugate acid of SO4–2. If you add H+ to SO4–2, you get HSO4-. TABLE OF CONJUGATE ACID-BASE PAIRS Acid Base K a (25 oC) HClO 4 ClO 4 – H 2 SO 4 HSO 4 – HCl Cl– HNO 3 NO 3 – H 3 O + H 2 O H 2 CrO 4 HCrO 4 – 1.8 x 10–1 H 2 C 2 O 4 (oxalic acid) HC 2 O 4 – 5.90 x 10–2 [H 2 SO 3] = SO 2 (aq) + H2 O HSO Conjugate Acid-Base Pair: An acid either loses a proton or accepts a lone pair of electrons to form a species called its conjugate base. Similarly, a base either loses a lone pair of electrons or... Conjugate Acid-Base Pair: In Bronsted-Lowry acid-base theory an acid is a proton donor and a base is a proton acceptor. When a compound or ion acts as an acid/base in a reaction it produces a
[index] [7327] [9503] [7723] [6886] [1415] [8487] [7396] [2087] [7846] [9788]
In the Brønsted-Lowry definition of acids and bases, a conjugate acid-base pair consists of two substances that differ only by the presence of a proton (H⁺).... This chemistry video tutorial provides a basic introduction into acids and bases. It explains how to identify acids and bases in addition to how they react ... Use Bronsted Lowry Acid/Base Theory to identify conjugate acid base pairs.More free chemistry help at www.chemistnate.com 068 - Acid-Base EquilibriumIn this video Paul Andersen explains how acid-base chemistry can be understood in terms of equilibrium. Water is present in all a... In this past live tutoring session, I focused on Acid and Base Neutralization Reactions, Precipitation Reactions, and Molarity. Understanding how to use a so... This chemistry video tutorial explains the concept of acids and bases using the arrhenius definition, bronsted - lowry and lewis acid base definition. It al... Learn everything about Conjugate Acids and Bases. We explain this with the real world example of vinegar.At Fuse School, teachers and animators come together... This chemistry video tutorial shows you how to identify an ionic compound as acidic, basic, or a neutral salt. You need to know the 6 common strong acids su... Introduction to conjugate acids and bases. Created by Sal Khan.Chemistry on Khan Academy: Did you know that everything is made out of chemicals? Chemistry is... In the Brønsted-Lowry definition of acids and bases, an acid is a proton (H⁺) donor, and a base is a proton acceptor. When a Brønsted-Lowry acid loses a prot...
Copyright © 2024 m.kingbet90vip.site